Phosgene synthesis is a critical process in organic chemistry. This compound, COCl2, plays a vital role in producing various pharmaceuticals and agricultural chemicals. Its importance cannot be overstated. It serves as an acid chloride in many reactions. However, the process of phosgene synthesis requires careful handling.
In the lab, phosgene is often generated by reacting carbon monoxide with chlorine. This method highlights its potential hazards. Proper safety measures are crucial when working with phosgene. Many aspiring chemists may overlook this risk. The significance of understanding phosgene synthesis lies in its wide applications. From dyes to insecticides, phosgene is essential in many industries.
Ultimately, the complexities of phosgene synthesis remind us of the delicate balance in chemistry. While it offers numerous benefits, the associated dangers necessitate a conscientious approach. Ongoing research and education about phosgene can lead to safer practices. This will enhance its potential in organic chemistry while minimizing risks.

Phosgene, a colorless gas with a faint odor, plays a crucial role in organic chemistry. Its chemical structure consists of one carbon atom, two oxygen atoms, and one chlorine atom, represented as COCl₂. This simple yet effective structure allows phosgene to act as an intermediate in synthesizing various compounds, including isocyanates and polycarbonate plastics.
Its importance in the industry is evident. According to a report by the Chemical Abstracts Service, phosgene is utilized in producing around 30% of all isocyanates. These compounds are essential in manufacturing foams, coatings, and adhesives. However, despite its utility, phosgene is highly toxic. Proper handling and safety protocols are paramount to avoid accidents.
Tips: Always ensure your workspace is well-ventilated when working with phosgene. Use appropriate protective gear, as even small leaks can pose serious health risks.
Phosgene's reactivity can lead to challenges. Improper storage may result in degradation, impacting the quality of synthesized materials. Furthermore, monitoring the purity of phosgene is critical. Impurities may alter its reactivity, leading to unpredictable results in reactions. Being aware of these factors is essential for success in organic chemistry.
Phosgene was first synthesized in 1812 by Frederick William Mahler. This compound, COCl₂, gained attention for its utility in organic synthesis. Initially, its role was overshadowed by the dangers it posed. Many chemists recognized this duality.
Further advancements came in the 19th century. Researchers began to understand phosgene’s importance in producing isocyanates and carbamates. These compounds are essential for creating various pharmaceuticals and pesticides. Data from the American Chemical Society indicates that phosgene derivatives are involved in over 20% of pharmaceutical processes. This significant percentage showcases its value in the industry.
Despite its usefulness, phosgene remains a hazardous substance. Safety protocols must be followed meticulously to prevent accidents. Historical incidents revealed the need for strict regulations. Modern synthesis techniques are now aimed at minimizing risks while maximizing efficiency. This balance between safety and utility continues to challenge chemists today.
Phosgene synthesis plays a vital role in organic chemistry. Understanding its methods is crucial for chemists. Various techniques have emerged. Each method has unique advantages and challenges.
One common technique involves the interaction of carbon monoxide with chlorine. This reaction produces phosgene efficiently. However, it requires strict safety controls. The gas is toxic, and exposure can have severe consequences. Another method utilizes the reaction of phosgene precursors. This alternative can be safer but may yield lower purity.
Moreover, advancements in technology have led to more refined approaches. Continuous flow reactors are gaining attention. They allow for better control over reaction conditions. Yet, these systems can be complex and expensive to implement. It's essential to balance safety and efficiency. The pursuit of better methods contemplates the fine line between innovation and risk.
| Synthesis Method | Advantages | Disadvantages | Applications |
|---|---|---|---|
| Carbonyl Chloride Method | High yield and efficiency | Toxicity concerns | Synthesis of isocyanates |
| Phosgene Carbonylation | Versatile for various substrates | Requires careful control of conditions | Pharmaceutical synthesis |
| Synthesis from Diethyl Carbonate | Less hazardous than other methods | Lower yields | Polymer production |
| Chloroform and Phosgene | Simple setup | Environmental concerns | Chemical intermediates |
| Acid Chloride Method | High reactivity | Side reactions possible | Resin and plastic production |
Phosgene, a colorless gas, plays a crucial role in the organic synthesis landscape. It is primarily utilized in the production of isocyanates and other critical intermediates. In 2020, the global isocyanate market was valued at approximately $19 billion. The increasing demand for these compounds is driven by their application in polyurethanes, a material widely used in various sectors.
Phosgene's significance extends beyond mere synthesis. It serves as a building block in pharmaceuticals, agrochemicals, and dyes. Reports indicate that over 40% of new drugs developed rely on phosgene at some stage. However, using phosgene requires stringent safety measures. It is toxic and poses significant risks during handling. Industry professionals need to reflect on these safety aspects continually.
Moreover, phosgene's role in the chemical industry is relatively underappreciated. While many embrace its utility, the challenges it poses are worth considering. The continuous search for safer alternatives has begun, though they are not yet as effective. Achieving a balance between utility and safety remains an ongoing conversation among chemists.
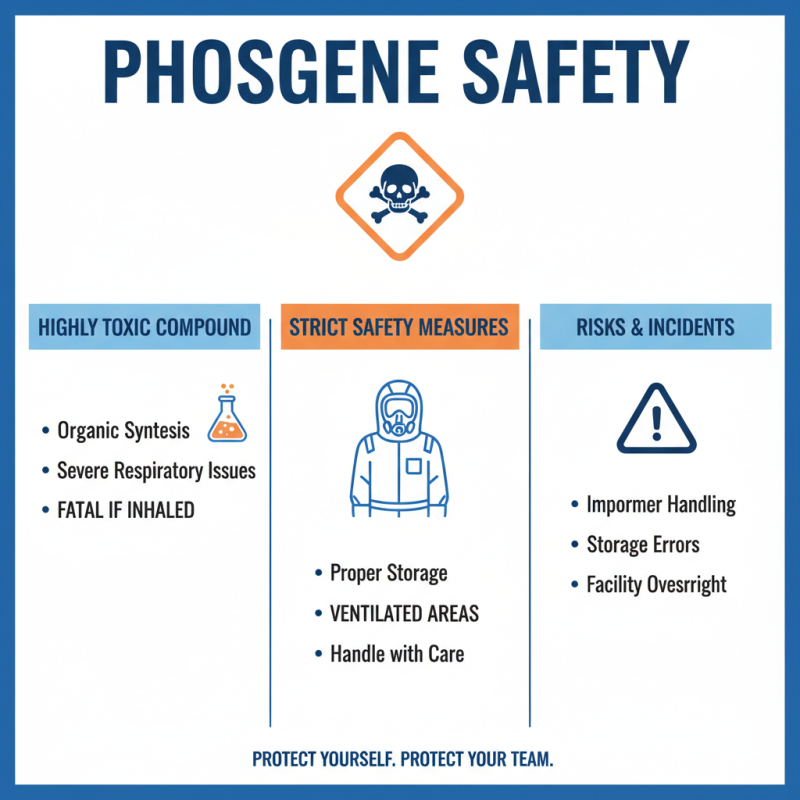
Phosgene is a highly toxic compound often used in organic synthesis. Handling this chemical requires strict safety measures. Exposure can lead to severe respiratory issues and even death. Industry reports indicate that incidents often arise from improper storage or handling procedures. In laboratories, phosgene must be stored in well-ventilated areas. However, many facilities overlook this critical aspect.
Safety protocols are vital. It’s alarming that data from safety audits show only 75% of labs comply with the recommended guidelines. Personal protective equipment (PPE) should be standard, yet some professionals underestimate its importance. Reports highlight that nearly 30% of accidents occur due to inadequate PPE usage.
Environmental factors also demand attention. Phosgene can contaminate air and water if not handled correctly. Studies have recorded phosgene emissions during manufacturing processes. Concerns about these emissions grow. Reducing environmental impact is essential for sustainable chemistry practices. Many organizations are now seeking alternatives, but the need for careful phosgene management remains.



Contact our team with questions, product inquiries or challenge us to engineer a solution for you.
Tel: +1 716 433 6764
Fax: +1 716 433 2850
Email: sale@ashymed.com
VanDeMark Chemical Inc.
One North Transit Road
Lockport, NY 14094 USA